

6.4 Important element trends down a Group. As you move from left to right across the periodic table, atoms have more electrons in their. 5.2 Period 3 element trends & explanations of physical properties. In Period 3, sodium with 11 protons is the least electronegative. Across a period from left to right, the covalent radius decreases. so electrons will be more strongly attracted to the nucleus. there are more electrons, but the increase in shielding is negligible because each extra electron enters the same shell. The acid-base behaviour of the "hydroxides" (using the term in the widest possible sense) of the Period 3 elements. Going across period 3: the nuclear charge increases. The physical properties of the chlorides of the elements from sodium to sulphur, their structure and bonding, and their reactions with water. Reactions of the oxides with water, and their acid-base behaviour. The first ionisation energy of the element increases across a period. The first ionisation energy is the amount of energy required to remove one electron from the outermost shell of each atom in one mole of atoms of an element in their gaseous state. The reactions of the elements from sodium to argon with water, oxygen and chlorine.ĭiscusses the link between the physical properties of the oxides of the elements from sodium to chlorine and their structure and bonding. First Ionisation Energy Trend Across Period 3. ĭiscusses the trends in first ionisation energy, atomic radius, electronegativity, conductivity, melting point and boiling point as you go across the period from sodium to argon.

3) Reactivity increases down the group- Due to increased shielding and. Nitrogen and phosphorus in group 15 usually form -3 ions.
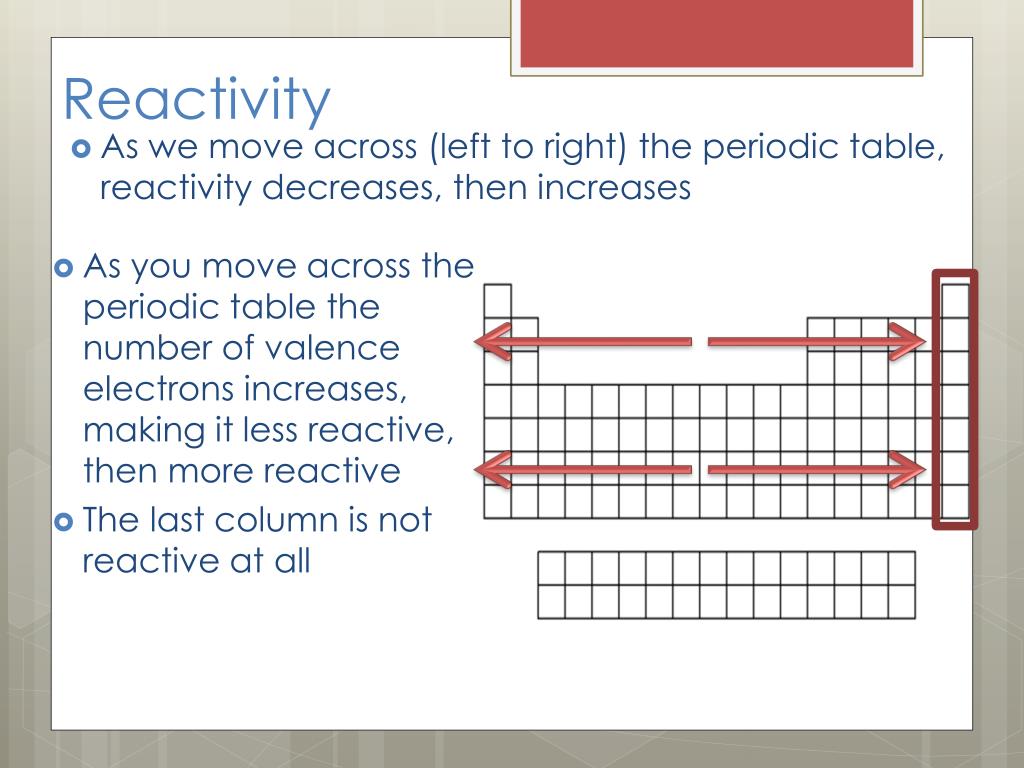
The alkaline earth metals always form +2 ions. Physical and atomic properties of the elements. What is the general trend in first ionisation energy ACROSS A PERIOD and WHY. ION CHARGE: - The number of electrons an element tends to gain or lose is a periodic property. 1.2.13 explain the trend in the first ionisation energies of atoms down Groups and across Periods in terms of nuclear charge, distance of outermost electron from the nucleus, shielding and stability of filled and half-filled subshells GCSE.


 0 kommentar(er)
0 kommentar(er)
